
This table compares the known valid mineral species listed listed with Chromium and the other elements listed based on the official IMA formula. Note that unlike other sections on this page this includes non-essential elements. The first data column contains the total number of minerals listed with Chromium and the element listed for that row. How many moles Chromium(III) Chloride in 1 grams? The final data column compares this percentage against the percentage of all minerals that contain the element listed in each row.›› More information from the unit converter The second data column lists this number as a percentage of all minerals listed with Chromium. Molecular weight of Chromium(III) Chloride or You can view more details on each measurement unit: We assume you are converting between moles Chromium(III) Chloride and gram. The molecular formula for Chromium(III) Chloride is CrCl3. The SI base unit for amount of substance is the mole.ġ mole is equal to 1 moles Chromium(III) Chloride, or 158.3551 grams. Note that rounding errors may occur, so always check the results.
CHEMICAL FORMULA FOR CHROMIUM III CARBONATE HOW TO
Use this page to learn how to convert between moles Chromium(III) Chloride and gram. ›› Details on molecular weight calculations ›› Common amount of substance conversions Grams Chromium(III) Chloride to moles, or enter other units to convert below: Enter two units to convert From: You can do the reverse unit conversion from ›› Quick conversion chart of moles Chromium(III) Chloride to gramsġ moles Chromium(III) Chloride to grams = 158.3551 gramsĢ moles Chromium(III) Chloride to grams = 316.7102 gramsģ moles Chromium(III) Chloride to grams = 475.0653 gramsĤ moles Chromium(III) Chloride to grams = 633.4204 gramsĥ moles Chromium(III) Chloride to grams = 791.7755 gramsĦ moles Chromium(III) Chloride to grams = 950.1306 gramsħ moles Chromium(III) Chloride to grams = 1108.4857 gramsĨ moles Chromium(III) Chloride to grams = 1266.8408 gramsĩ moles Chromium(III) Chloride to grams = 1425.1959 gramsġ0 moles Chromium(III) Chloride to grams = 1583.551 grams Type in your own numbers in the form to convert the units! Chromium iii carbonate formula how to#
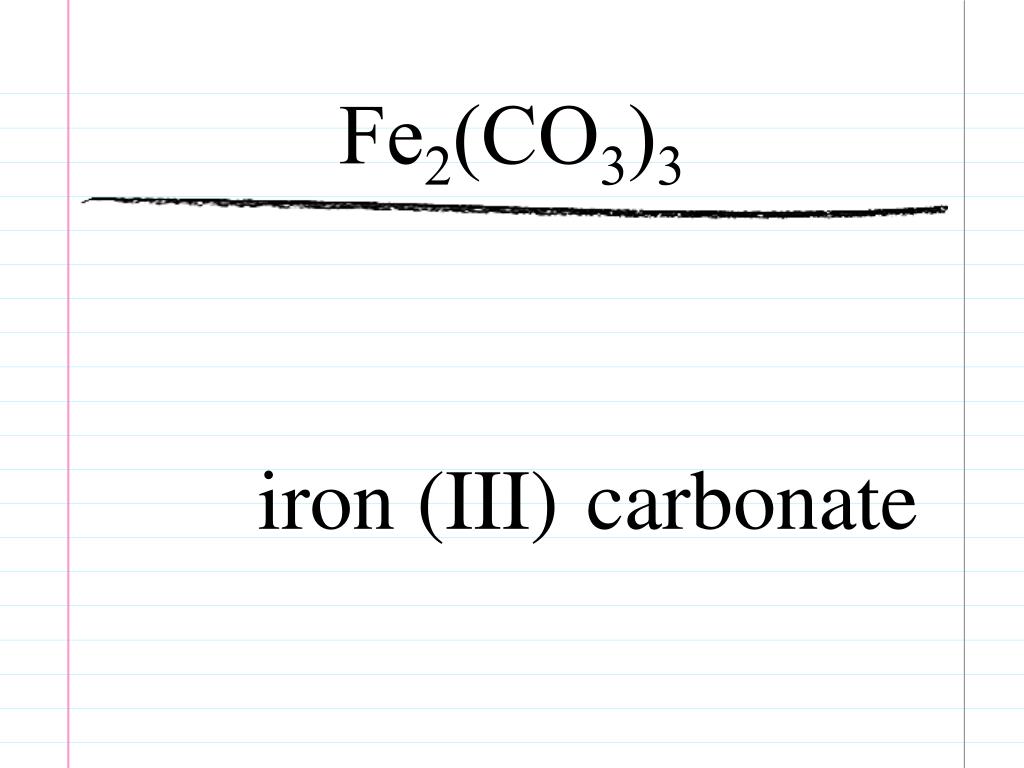
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.Ī common request on this site is to convert grams to moles.


 0 kommentar(er)
0 kommentar(er)
